Abstract
Background: Pulmonary hypertension (PH) is associated with premature mortality in adults with sickle cell anemia (SCA). Decreased nitric oxide bioavailability has been implicated in the pathogenesis of SCA associated PH. Thrombospondin-1 (TSP1) protein encoded by THBS1 gene inhibits nitric oxide/cGMP signaling and has been hypothesized to promote PH in SCA (Novelli et al. Am J Physiol Lung Cell Mol Physiol 2019). Recently in a cross-sectional targeted gene analysis in adults with SCA, THBS1 gene single nucleotide polymorphisms (SNPs) rs1478605-G and rs1478604-T were found to be associated with PH, as estimated by its surrogate echocardiogram marker tricuspid regurgitant velocity (TRV). However, neither of these SNPs were validated in an external cohort (Jacob et al. Am J Hematol. 2017).
Objective: To test the hypothesis that THBS1 gene SNPs were associated with TRV, we first validated the association of previously reported THBS1 gene SNPs (rs1478605-G and rs1478604-T) with TRV as quantitative and binary variables (elevated TRV: < or ≥ 2.5m/sec) in a longitudinal, pediatric, SCA patient cohort. We then assessed the associations of 8 other common THBS1 SNPs with false discovery rate controlled at 0.1. Finally, we sought to generate a polygenic score (PGS) incorporating this region genomic data and determine its association with TRV.
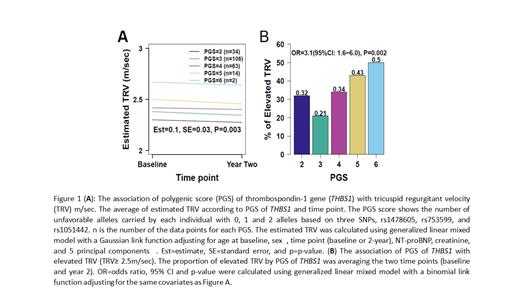
Methods: Our cohort was comprised of children aged 5-18 years with HbSS or HbS/β 0thalassemia enrolled in either Long-Term Effects of Erythrocyte Lysis Trial (ELYSIS, NCT00842621) (Yates et al. Pediatr Blood and Cancer. 2019) and Sickle Cell Clinical and Intervention Program (SCCRIP, NCT02098863). All participants underwent prospective measurement of TRV by 2D-echocardiogram at baseline steady state (≥ 4 weeks from transfusion, hospitalization) and then repeated two years later (Rai et al. Blood Adv. 2021). Generalized linear mixed models were used to assess the association between quantitative or elevated TRV and genetic markers, after adjusting for age at baseline, sex, time point, NT-pro-BNP, creatinine and 5 principal components (PC). Three SNPs, rs1478605-G, rs753599-A and rs1051442-T statistically significant at levels given above for either quantitative or elevated TRV and whose pairwise linkage disequilibrium R 2 ≤ 0.1 were selected to build a PGS. The PGS was defined as the summed number of TRV-increasing unfavorable alleles carried by each individual across all selected SNPs. Two-stage iterative resampling (TSIR) approach was used to internally discover and validate the associations of PGS with TRV at an overall type I error rate of 0.05 with randomly sampled half of samples as the discovery cohort and the remaining as the validation cohort (Kang et al. J Hum Genet. 2015).
Results: Our cohort included 138 children (276 data points) with SCA with mean age of 9.9 years (SD 3.25 years). We validated the association of quantitative TRV with previously reported two SNPs rs1478605 (estimate = -0.11, standard error [SE] = 0.04, p = 0.003) and rs1478604 (estimate = -0.09, SE = 0.04, p = 0.014). We further identified new associations of elevated TRV with THBS1 SNPs rs10551442 odds ratio (OR) 0.26 (95% CI 0.09, 0.77; p = 0.017), rs17633107 OR 0.26 (95% CI 0.09, 0.77; p=0.017), rs3743125 OR 4.4 (95% CI 1.2, 1.7; p = 0.03), and rs753599 OR 5.3 (95% CI 1.4, 2.1; p=0.018). Two of these SNPs rs10551442 (estimate = -0.13, SE = 0.05, p = 0.013) and rs17633107 (estimate = -0.13, SE = 0.05, p = 0.013) were also associated with quantitative TRV. One unfavorable allele increase in PGS was associated with a 0.1 m/sec increase in TRV (Figure 1A) and a 2.1 increase in log-odds of having an elevated TRV (Figure 1B). The associations of PGS with quantitative and elevated TRV were discovered and validated in 36 and 21 out of 100 repetitions by the TSIR analysis, respectively.
Conclusion: We validated two previously published variants in the THBS1 gene rs1478605 and rs1478604 in an external and independent pediatric cohort with quantitative TRV. We also identified associations of common SNPs rs10551442, rs17633107, rs3743125, and rs753599 with TRV ≥ 2.5m/sec and of rs10551442 and rs17633107 with quantitative TRV in a pediatric population and created a PGS incorporating these data. The PGS is significantly associated with TRV, which was internally validated using TSIR approach. Our genomic findings further support the role of TSP1 in the pathophysiology of the pulmonary vasculopathy seen in SCA.
Ataga: Forma Therapeutics: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Consultancy; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Agios Pharmaceuticals: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees. Hankins: Global Blood Therapeutics: Consultancy; Vindico Medical Education: Consultancy; Bluebird Bio: Consultancy; UpToDate: Consultancy. Estepp: Global Blood Therapeutics: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal